Improvements in periodic representation of solvated systems with FHI-aims
ARCHER2-eCSE08-03
PI: Dr Andrew Logsdail (Cardiff University)
Co-I(s): Dr Gabriel Bramley (Cardiff University), Prof Reinhard Maurer (University of Warwick), Dr Volker Blum (Duke University), Prof Dr Harald Oberhofer (University of Bayreuth), Dr Jakob Filser (Fritz Haber Institute Berlin)
Technical staff: Dr Pavel V. Stishenko (Cardiff University)
Subject Area:
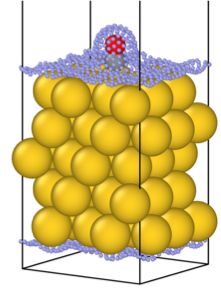
The Au(111) slab (yellow spheres) with adsorbed CO molecule (black and red spheres). Small blue spheres represent the point mesh of solute-solvent interface built by the MPE model. Black lines represent the unit cell.
Computational modelling gives invaluable insights into chemical processes at the atomic scale. These insights help to develop new catalysts for carbon-neutral production chains, new adsorbents for medicine and environment protection, and new sustainable materials.
Many processes that are relevant for this chemistry occur in the liquid phase near the solid surface; e.g., chemical reactions on metal catalysts, adsorption of contaminants from water in porous materials, and material degradation/corrosion. Atomistic modelling of liquids is a notoriously difficult task due to the complexity of the molecular interactions and configurations, so several implicit models have been developed that incorporate a balance of accuracy and computational cost. This eCSE project has enabled the implementation of new infrastructure to study solid materials in implicit liquid solvents, using periodic boundaries to simulate the macroscopic scale of the system.
FHI-aims is a widely adopted code for quantum chemistry and materials science. It is especially efficient (and popular) for simulation of solid surfaces, due to their chemical importance. Throughout this project, the capability of FHI-aims to simulate solid surfaces within liquids has been improved. Most notably, an implicit solvent model based on the multipolar expansion of the solvent’s electrostatic potential has been implemented for periodic systems, with foundations laid for further performance optimizations. Additionally, infrastructure has been formalized to calculate corrections for basis set superposition errors over all supported levels of theory, including hybrid and double hybrid density functional approximations.
The figure showcases the new kind of models supported by FHI-aims. This is a carbon monoxide molecule adsorbed on a gold surface – a well-studied system, that signified a breakthrough in catalysis on nanoparticles. In the majority of atomistic simulations, such a calculation would be performed in a vacuum, without a solvent. But here, the mesh of blue dots shows the boundary of the implicitly represented liquid solvent, that can now be taken into account, providing closer alignment of computer models with real-life systems.
The work presented improves the capability of an active software package on ARCHER2, improving usability and performance to the benefit of users. The infrastructure updates also deliver benefits for long-term sustainability, and present opportunities to extend functionality in novel and valuable ways.
Information about the code
The FHI-aims code is available on ARCHER2 via the modules infrastructure:
module load fhiaims